Glossary of Nanotech
Measurements
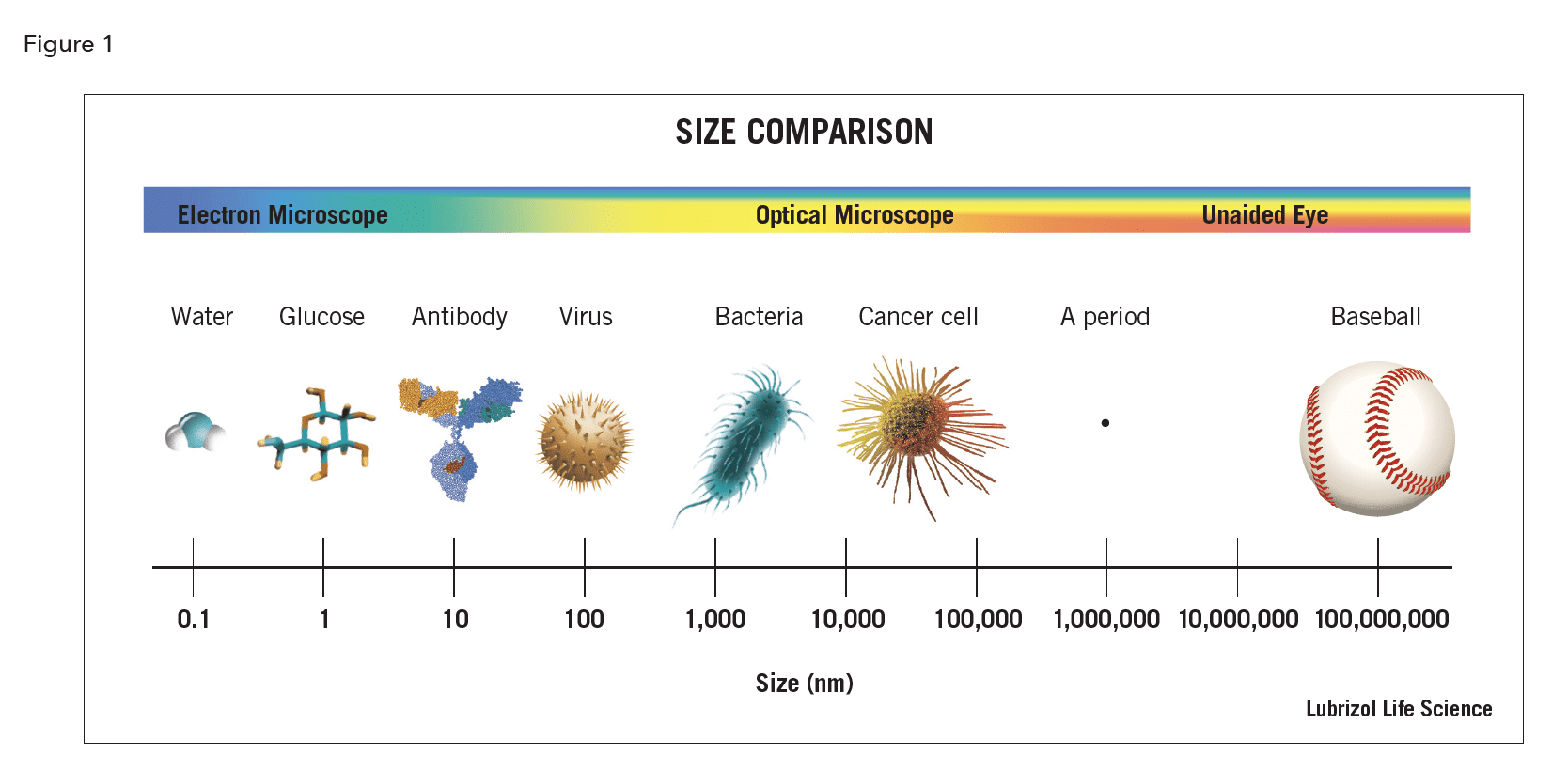
Angstrom (Å) – a unit of length equal to one ten billionth of a meter or one tenth of a nanometer. Its symbol is the Swedish letter Å. It’s most often used a measurement of atoms and molecules.
Micron (μm) – a metric system unit of length equal to one millionth of a meter. Its symbol is µ, the Greek letter mu and is often written as μm. Most cells, hair, and drug particles are measured in microns.
Molecular mass – the sum of the mass of the atoms that make up a given molecule. The term molecular weight is often incorrectly used interchangeably with molecular mass. The most common unit used is the kD (kilodalton). A kilodalton is 1000 Da (dalton). 1 Da equals the mass of 1 hydrogen atom.
Molecular weight – the average weight of a molecule, element, or compound measured in units once based on the weight of one hydrogen atom or alternatively, on 1/16 the weight of an oxygen atom, but, after 1961, now based on 1/12 the weight of a carbon atom.
Nanometer (nm) – a metric system unit of length equal to one billionth of a meter, abbreviated nm. 1000 nm = 1 μ = 0.001 millimeter. Viruses and drug nanoparticles are typically 10s to 100s of nm in diameter.
Surface area – the area of exposed surface of a given object. For nanoparticles, it is usually measured in meters2. Irregular or porous surfaces will have greater surface area compared to smooth objects of same overall dimensions. Higher surface area particulates will generally dissolve faster than lower surface area particles of the same material.
Techniques
Molecular weight determination
- Size-exclusion chromatography (SEC) – is a chromatographic method in which molecules in solution are separated by their size or molecular weight. It is usually applied to large molecules including proteins and polymers. SEC is a widely used polymer characterization method because of its ability to provide good molecular weight distribution results for polymers.
Particle size analysis – the particle size distribution (PSD) of a powder, granular material, or particles dispersed in fluid is a list of values or a mathematical function that defines the relative amount (typically by mass/volume) of particles present according to size. Knowledge of the PSD of a material is important in research, product development, processing, handling, packaging, and quality control. The PSD is needed to understand the physical and chemical properties of a suspension. The PSD is usually defined by the method by which it is determined.
- Acoustic attenuation spectroscopy (AAS) – the attenuation and speed of ultrasound pulses as they pass through concentrated slurries (>60 weight %) are measured over a wide range of frequencies. The resulting spectra are used to calculate the PSD over a size range, depending upon density, from 10 nm to 100 µ. AAS is an ensemble averaging technique of fairly low resolution. Unlike optical methods, AAS is inherently very robust and not sensitive to fouling and therefore suitable for online production monitoring and control, including application in process analytical technology.
- Disc centrifuge photosedimentometry (DCP) – a disc centrifuge photosedimentometer operates using the principle of Stokes’ law. A small particle moves more slowly through a fluid than a larger one. Given that the particle density is known, timing the descent of a particle through a fluid will, when Stokes’ law is applied, give the size of the particle. Depending on density, DCP can analyze particles ranging in size from approximately 5 nm to 100 µm and it provides a true weight average PSD directly without calibration. Resolution is extremely good; typically peaks in a multimodal PSD differing in mean size by as little as 5% can be resolved. The maximum suspension concentration is typically around 2 volume %.
- Dynamic light scattering (DLS) – also known as photon correlation spectroscopy (PCS), quasi-elastic light scattering (QELS), or intensity fluctuation spectroscopy (IFS) – is a technique used to determine the PSD of small particles (typically less than 1 µ) in very dilute suspensions or low concentration polymer solutions. A beam of laser light is passed through the suspension being examined and the intensity of the light scattered from the particles measured (conventionally at 90 degrees to the incident beam but also, in some configurations, “backscattered” at approximately 170 degrees). DLS is an ensemble averaging technique of fairly low resolution.
- Field flow fractionation (FFF) – a “force field” is applied to a particle suspension through a long and narrow channel, perpendicular to the direction of flow, to cause separation of the particles present in the fluid. It is dependent on their differing “mobilities” under the force exerted by the field. The force field can be electrical, gravitational, centrifugal, magnetic, or thermal-gradient. The particle size resolution is similar to DCP. The size range varies depending upon the FFF configuration from 1 nm up to 100 µm. FFF can also be used in the separation of macromolecules and proteins.
- Laser diffraction analysis (LDA) or laser diffraction spectroscopy (LDS) – utilizes the properties of the diffraction patterns of a laser beam passed through a suspension to measure the PSD of its particles. It is based on the theory of Fraunhofer diffraction, which states that the intensity of light scattered by a particle is directly proportional to the size of the particle (typically greater than 1 µm). In LDA, the laser is passed through the suspension being examined and the diffracted light focused onto a detector from which the angular distribution of the intensity of the scattered light is measured. LDA is an ensemble averaging technique of fairly low resolution.
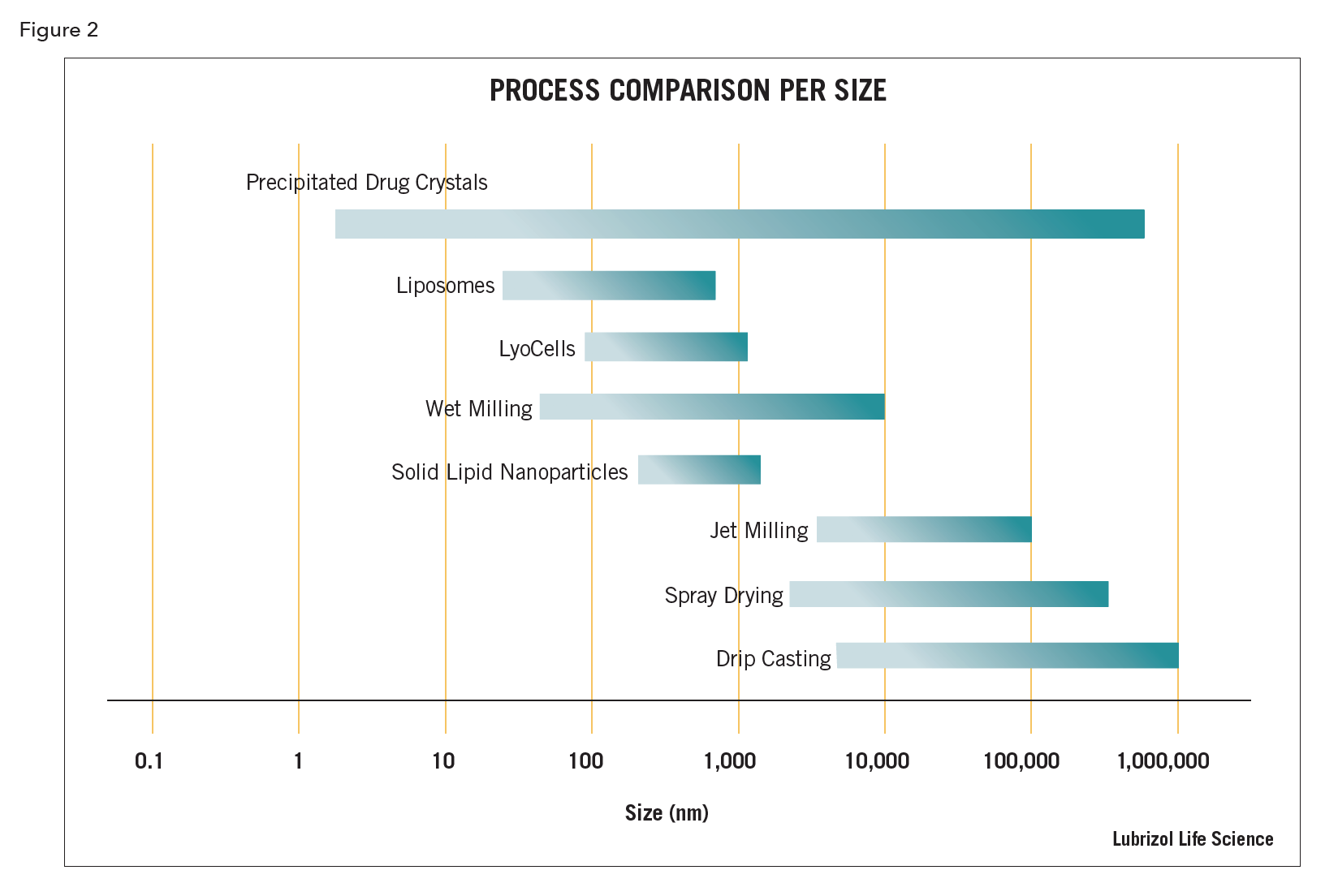
Particle size reduction – commonly applied to a poorly soluble active pharmaceutical ingredient (API) to increase its bioavailability. The reduction in particle size results in a dramatic increase in surface area, which in turn increases the material’s dissolution rate and thus “apparent” solubility. Particle size reduction may also be performed to increase homogeneity within a dosage form, especially for those forms with low API loading. Both wet and dry techniques exist, although the wet techniques are generally capable of resulting in smaller particle sizes
- High shear homogenization – a technique where a suspension of API (or other material) is pumped under very high pressure through a narrow channel, gap, or orifice. The particle size is reduced by the generation of high shear forces via cavitation and/or impingement. A number of designs exist for the shear generation zone, including micro-channel arrays, piston gap valve, and nozzles, with some designs also incorporating cross current fluid on fluid impingement. High shear homogenization can also be used for size reduction of emulsion, micelle, or liposomal systems.
- Media milling – advancement on the roller milling technique wherein, rather than relying on the tumbling of the milling media under the force of gravity, the media is moved by a rotating agitator. Media milling may be performed either in a distinct batch mode or, more normally, in a flow-through continuous mode, wherein the suspension is pumped through the milling chamber for one or multiple passes until the particles are reduced to the target size. Media mills are available in both horizontal and vertical configurations. Due to the high speeds at which the agitators rotate, this is generally a high energy process.
- Recrystallization – a technique of dissolving the API or material in a solvent, followed by alteration of conditions to affect reprecipitation into crystalline or sometimes amorphous nanoparticulates. Conditions to cause precipitation are normally by addition of or addition into a miscible non-solvent for the dissolved material, but could also include adjustment of conditions such as pH or temperature. Manipulation of conditions including solute concentration, solvent/non-solvent ratio, and/or rate of addition is critical to achieve nanoparticulates. Recrystallization may also be conducted under high shear conditions to limit crystal size growth during the process and there are machines specifically designed for this purpose (high shear homogenizers and ultrasonic processors).
- Roller milling – a technique wherein particle size reduction is affected by the addition of a suspension of the material in a non-solvent vehicle into a jar or vessel also containing milling media (normally spheres or sometimes cylinders of dense wear-resistant material like ceramic). Milling occurs by rolling the vessel and the resultant cascading action of the media grinding the suspended particulates to smaller sizes. Roller milling is a low energy process.
- Ultrasonics – size reduction of emulsions, liposomes, and solid particulates may be affected by ultrasonic processing. Ultrasonic equipment is defined by frequencies and power densities. These systems generate intense cavitation which induces high levels of shear to the materials being processed. Ultrasonic processors are available in multiple configurations from simple probes that are immersed into the liquid for small batch preparation, to complex flow-through units capable of commercial production quantities.
Stabilization
- Electrostatic – a mechanism for stabilizing particle suspensions against aggregation based on the mutual repulsion of like electrical charges. The charge can be positive (cationic) or negative (anionic) and can be inherent (i.e., charge on oxide surfaces) or induced by adsorption of charge-modifying agents such as tensides and polyelectrolytes. Electrostatic stabilization is affected by addition of salt (electrolyte) to a suspension or changing the pH of a suspension.
- Steric – a mechanism for stabilizing particle suspensions against aggregation by covering particles with polymer(s) which prevents particles from getting close to each other and within the range of attractive forces; it is both polymer molecular weight (MW) and concentration dependent. Some degree of steric stabilization can be obtained using non-ionic surfactants. The advantage of steric stabilization is that it can infer stability in non-aqueous media and protect against aggregation in aqueous suspensions at high electrolyte levels.
Zeta potential determination
Zeta potential (ZP) is a term used to describe the electrokinetic potential in colloidal systems. The ZP is the electric potential in the interfacial double layer at the location of the slipping (or shear) plane; it is the potential difference between the dispersion medium and the stationary layer of fluid attached to the dispersed particle. The significance of ZP is that its value is a surrogate for the surface charge (although the exact relation is complex and non-linear) and can be related to the stability of colloidal dispersions. The ZP indicates the degree of repulsion between similarly charged particles in a dispersion. ZP is not measurable directly but it can be calculated using theoretical models and an experimentally-determined electrophoretic mobility. Electrokinetic phenomena (electrophoresis, electro-osmosis, sedimentation potential, and streaming potential) and electroacoustic phenomena (colloid vibration potential and electrokinetic sonic amplitude) are the usual sources of data for calculation of ZP.
- Electroacoustic attenuation (EAA) – allows measurements to be made in concentrated suspensions without dilution, more appropriate to practical formulations. EAA is a variance on sedimentation potential where gravitational force is replaced by an acoustic field. Importantly, the sample under investigation need not be stationary, which is a requisite of ELS and PALS. The dynamic particle size range that can be studied ranges from a few nm to tens of μm. A disadvantage of EAA is interference from the presence of air bubbles.
- Electrophoretic Light Scattering (ELS) – microelectrophoresis is the most widely used of the electrokinetic phenomena. ELS is based on microelectrophoresis but utilizes light scattering (time or frequency domain analysis) to detect particle motion. ELS provides a histogram of the ZP of the particles within the sample suspension; the technique usually requires dilution of the sample, which might affect properties of the sample and change ZP.
- Phase Analysis Light Scattering (PALS) – is similar to ELS except that it utilizes phase modulation instead of frequency spectrum analysis. The method is ideal for non-aqueous suspensions, for aqueous suspensions in physiological saline conditions and for particles that are (sterically) stabilized by adsorbed nonionic surfactants. It can only provide an average value for ZP, i.e., there is no histogram.
- Streaming Potential (SP) – is useful for materials of massive size or irregular shape, such as hair, bone, skin, fibers, membranes, and filters. A major advantage of the SP technique is the ability to investigate adsorption/desorption phenomena in situ; the flow-through nature of the method makes it ideal for the study of long-term processes. The use of material of macroscopic dimensions often provides data which is more appropriate to process conditions. Only an average value for ZP can be obtained. Due to technical issues (such as electrode polarization) measurements are limited to electrolyte solutions of <10-2
Types
Block copolymer micelles (BCMs) – block copolymers like those used to prepare polymersomes can also form micelles in aqueous solution. The hydrophobic blocks of the individual polymer chains aggregate to form the core of the micelles and the hydrophilic blocks extend from the core into the aqueous phase. There they are solubilized and provide stabilization against further growth and aggregation. The core of BCMs can be loaded with hydrophobic drugs, and, if made of biodegradable polymers, can release the drug over time by hydrolysis of the particle.
Cubosomes (see LyoCells®) – nanoparticles of bicontinuous cubic liquid crystalline phase. The aqueous domains form interpenetrating networks with the hydrophobic domains, and, as such, LyoCells and cubosomes can encapsulate both hydrophobic and hydrophilic drugs and proteins.
Liposome – spherical nanoparticles made of one or more phospholipid bilayers surrounding an aqueous core. Liposomes are used to encapsulate water-soluble APIs in the core, oil-soluble APIs in the bilayer membrane, and, if made of cationic phospholipids, trap and deliver oligonucleotides for gene therapy.
LyoCells® – reverse cubic phase lipidic nanoparticles with hydrophobic and hydrophilic domains that are never more than a few nanometers apart, giving the particles unique solubilization properties.
Micellar nanoparticles® – multiphasic compositions consisting of API distributed in micelles, nanoemulsion, crystalline (macro and nano), and continuous phase. These formulations are used for multiple routes of administration, including transdermal delivery.
NanoCrystal Colloidal Dispersions® – crystalline, high melting, poorly water-soluble API in the nanometer srange (<400 nm) that are stabilized in aqueous media. Stabilizers can be either non-ionic, ionic, or a combination of both.
Polymeric nanoparticle (PNP) nanoparticles made of solid polymers, by top down or bottom up processes, such as solvent displacement. Typically, a surfactant is used to stabilize the particles, but PEGylated polymers can be from self-stabilizing PNPs. Like SLNs, PNPs are useful for encapsulating hydrophobic drugs or entrapping biologics on the surface. If the polymers are biodegradable polyesters, drugs can be released from the particle over time by degradation of the particle in vivo.
Polymersomes – similar to liposomes, except that instead of using phospholipids to make the bilayers of the nanoparticle, synthetic block copolymers with a hydrophobic block and a hydrophilic block are used.
Quantum dots (Q-dots) – nanoparticles that are a few nm in diameter and made from semiconductor materials, such as cadmium selenide. The electronic properties of Q-dots are determined by the particle size, and hence the optical properties of their dispersions (such as color) depend on particle size. Q-dots are used in diagnostics and imaging.
Solid lipid nanoparticle (SLN) – nanoparticles made of lipids that melt above room temperature, usually made by bottom-up methods such as melt-emulsify-cool methods or solvent displacement. Useful for encapsulating hydrophobic drugs that are soluble in the molten lipid and don’t recrystallize from it upon cooling/hardening. Common lipids are carnauba wax, lecithin, cholesterol, bees wax, emulsifying wax, Compritol, and Dynasan. The nanoparticles are stabilized in aqueous dispersion by an adsorbed surfactant. If the surfactant is electrically charged, proteins, peptides, and oligonucleotides can be electrostatically attached to the surface for vaccination and gene therapy.
Misc
Agglomeration – is a process whereby aggregate particulate structures in a suspension stick together to create larger particulate complexes. Agglomerates are generally amenable to redispersion and are formed when aggregate suspensions settle or clump over time or are induced by the addition of chemical additives (coagulants and flocculants). Agglomerates are the larger size tail in any particle size distribution (PSD). The presence and structure of agglomerates directly affects suspension properties such as rheology.
Aggregation – is a process whereby individual (primary) particles in a suspension stick together to create particulate structures. Aggregates are formed when dispersions are dried; the forces involved are such that it is difficult and often impossible to reconstitute the original dispersion. Aggregates tend to be the major constituent fraction of any PSD.
Amorphous – an amorphous substance has no definite shape (or is irregularly shaped) or is formless and of no recognizable character. Importantly, an amorphous solid lacks the ordered structure of crystals; while there may be local ordering of the atoms or molecules in an amorphous solid, no long-term ordering is present.
Crystalline – a crystalline substance is a solid material whose constituent atoms, molecules, or ions are arranged in an ordered pattern extending in all three spatial dimensions. In addition to their microscopic structure, large crystalline solids are usually identifiable by their macroscopic geometrical shape, consisting of flat faces with specific, characteristic orientations.
Dispersant – a true dispersant (also known as a plasticizer) is a non-surface-active substance added to a suspension, usually a colloid, to improve the separation of particles (usually by electrostatic forces), or to maintain the dispersed particles in suspension and prevent clumping over time.
Oligomer – is a molecule that consists of a relatively small and specifiable number of monomers (usually less than five). Unlike a polymer, if one of the monomers is removed from an oligomer, its chemical properties are altered.
Oswald ripening – is the phenomenon in which small particles (less than 1 µm) of very sparingly soluble materials dissolve and then subsequently recrystallize onto larger particles so the system can attain a more thermodynamically stable state (i.e. lowering overall energy since smaller particles have a higher surface energy, hence a higher total Gibbs free energy). It does not occur for substances that are either completely soluble or are completely insoluble in a given medium. This shrinking and growing of particles will result in a larger mean diameter of a PSD over time. Ostwald ripening affects nucleation and precipitation processes and can lead to destabilization of dispersions and emulsions.
PEGylation – PEG is short for polyethylene glycol, a water-soluble polyether that is often used as a dispersion stabilizer in food and drug products. PEGylation is the process of chemically conjugating PEG to a substance such as a hydrophobic drug molecule to increase its water solubility, or a protein molecule to reduce aggregation, or to a nanoparticle to make it “stealthy”, thereby avoiding clearance from the blood by macrophages and dendritic cells.
Polymer – any of the numerous natural and synthetic compounds of typically high MW, consisting of up to millions of repeated, linked units, each a relatively light and simple molecule. A polymer can be a three-dimensional network (repeating units linked together left and right, front and back, up and down) or a two-dimensional network (repeating units linked together left, right, up, and down in a sheet) or a one-dimensional network (the repeating units linked left and right in a chain). Polymers can be crystalline or amorphous. A polymer of two or more different monomers is termed a copolymer and such materials have an array structure. A block copolymer consists of macromolecules in which comparatively long sequences of the links of one monomer (blocks) alternate with blocks of another monomer.
Surfactant – the term “surfactant” is a shortened form of “surface-active agent”. A surfactant (also called a tenside) is a chemical that reduces the surface tension at the interface between water (or other fluid) and air or the interfacial tension between two fluids. Surfactants are compounds that are amphiphilic, meaning they contain both hydrophobic groups (their tails) and hydrophilic groups (their heads). They can be cationic, anionic or nonionic and they perform one or more functions including serving as detergents, wetting agents, foaming agents, emulsifiers, conditioning agents, and solubilizers.
