Nanotechnology – New Name – Old Science
A colloid is defined as a homogeneous, non-crystalline substance in which insoluble particles are dispersed in another substance. Colloids are present in many modern, everyday products, such as pharmaceuticals, cosmeceuticals, and foodstuffs, but have also been a part of human life for thousands of years. Stone Age paintings in the Lascaux caves of France were produced with stabilized colloidal pigments, ancient Babylonian tablets describe the preparation of colloidal inks, and Egyptian hieroglyphs depict silt (a colloid) on the Nile delta.
Colloidal particles range in diameter from 1-1000 nanometers and can be solid, liquid, or gaseous. Our discussion here will be limited to particles within the 1-100 nanometer scale, better known as nanoparticles.
Nanoparticles have become an increasingly important and popular technology in many parts of modern society. We see these systems in textiles, computer systems, and, most importantly for us, pharmaceuticals. The first nanoparticle-based pharmaceuticals were approved in the 1990s, and in 2007, the FDA issued its Nanotechnology Task Force Report, defining nanotechnology as the ability to measure, see, manipulate, and manufacture things usually between one and 100 nanometers. Nano-size particles are simply a new term for colloids.
In the years since, multiple nanoparticle-based pharmaceuticals have been approved, and nanoparticles are still being heavily investigated for targeted drug delivery, solubility and bioavailability enhancement, and controlled therapeutic release.
The Importance of Particle Properties
When a colloidal dispersion is formed, an interface is created between the particles and the dispersion medium1. The presence of an interface leads to effects such as capillarity, which is the tendency of the medium to move as a result of tension, and adsorption, which is the process by which particles adhere to a surface. These two phenomena play a significant role in how colloidal dispersions behave in drug delivery systems, and are strongly influenced by specific properties of the system particulates, including:
- Particle size
- Particle shape
- Surface charge
Particle Size
The particle size distribution (PSD) directly affects the bioavailability of active pharmaceutical ingredients (APIs) and the safety of intravenous lipid emulsions. As with suspensions, colloidal dispersions also contain a range of sizes which define the PSD.
Two procedures are used for the preparation of colloidal dispersions4,5. In the dispersive, or “top-down” method, particles are distributed in the medium by comminution and attrition with various milling techniques. With this method, the average particle size can be made quite small, but the PSD is usually broad. In the condensation, or “bottom-up”, method, individual molecules are made to combine to form aggregates. The resulting PSD is much narrower than the dispersive method, but dispersions formed this way are subject to Ostwald ripening—a process by which smaller particles redeposit onto larger ones over time.
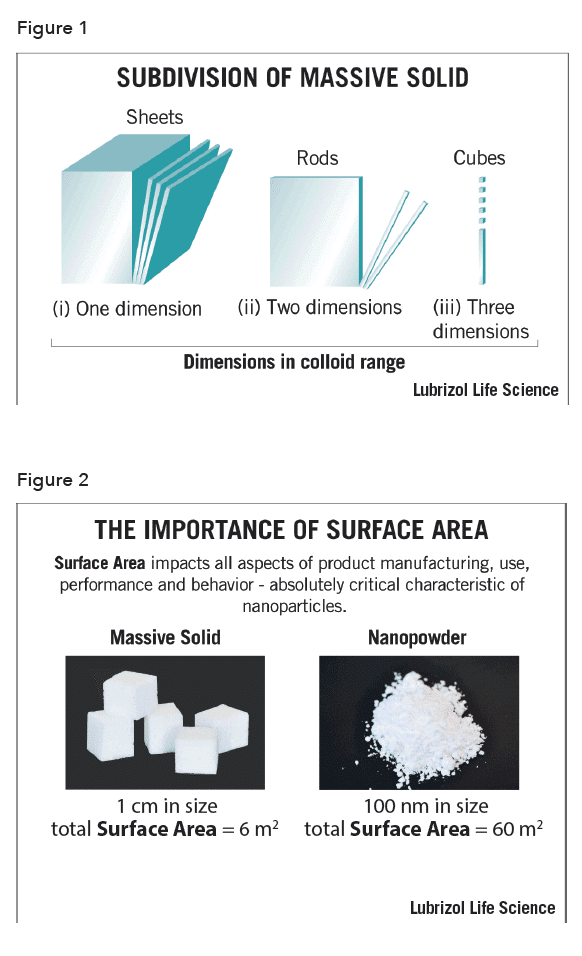
When the particle size of an API is reduced, the surface area increases exponentially (see Figure 1) and the particles exhibit a higher surface-area-to-volume ratio. More surface area allows smaller API particles to have greater interaction with surrounding water and a higher dissolution rate in the human body. Figure 2 demonstrates how turning a 1 cm2 cube into 100 nm nanoparticles results in a 100,00-fold increase in surface area. The same logic applies to dissolving sugar cubes vs. powdered sugar. Because the powdered sugar has significantly higher surface area, it dissolves much faster in tea or coffee.
 Additionally, as material is broken down, the internal surface becomes exposed bringing a change in the number or type of surface chemical sites and groups. Referring again to our 1 cm2 particle (cube), only two or three molecules in 10 million are “surface” molecules. However, when divided into 10 nm size particles, more molecules/atoms that comprise the molecular structure become “surface moieties” and the ratio rises to nearly 1:4. At 1 nm particle size, fully 80% of the atoms are on the surface. Large increases in surface area will also affect the interaction between particles and system properties, such as suspension rheology, coating, and adhesion. In general terms, smaller particles dissolve more quickly and, therefore, exhibit higher bioavailability.
Additionally, as material is broken down, the internal surface becomes exposed bringing a change in the number or type of surface chemical sites and groups. Referring again to our 1 cm2 particle (cube), only two or three molecules in 10 million are “surface” molecules. However, when divided into 10 nm size particles, more molecules/atoms that comprise the molecular structure become “surface moieties” and the ratio rises to nearly 1:4. At 1 nm particle size, fully 80% of the atoms are on the surface. Large increases in surface area will also affect the interaction between particles and system properties, such as suspension rheology, coating, and adhesion. In general terms, smaller particles dissolve more quickly and, therefore, exhibit higher bioavailability.
Overcoming poor bioavailability is one of the greatest challenges in the pharmaceutical industry, as it can result in ineffective treatments, higher expenses for patients (since more drug amounts would need to be purchased), and unpredictable drug delivery, which increases the risk of toxic side effects. Particle size reduction has been applied to numerous BCS Class II and IV APIs, which have notoriously low bioavailability, leading to multiple FDA approvals since the year 2000.
Particle Shape
Particle asymmetry is also a factor of considerable importance in determining the overall properties of colloidal systems. Shape is a function of the history of the formation of the particles, i.e., crystallization4. Although the exact shape may be much more complex, colloid particles can be roughly classified as: corpuscular (spherical and ellipsoid), laminar (disc- or plate-like), linear (rod- or needle-like), or random coil, which is typical of globular proteins such as albumin and globulin5.
Many APIs exist as rod- or needle-like particles. High molecular weight materials, such as proteins, polysaccharides and synthetic polymers, usually exist in the form of long thread-like or branched-chain molecules. These materials often exhibit a considerable mechanical strength and durability not possible with corpuscular or laminar particles6. Their shape is influenced by solution conditions such as temperature, pH, salt/electrolyte concentration, and range in configuration7. This determines if they will act as either a stabilizer or a de-stabilizer of a particulate dispersion.
Surface Charge
As a colloidal system is broken down in the body, the suspended particulate surfaces become increasingly exposed, resulting in a change in the number or type of surface chemical sites and groups. Thus, at the interfaces between the disperse phase and the dispersion medium, electrical (surface charge) effects can result and influence system behavior. The mechanisms by which this can occur are8:
- Affinity differences of two phases for electrons
- Ionization of surface groups
- Differential ion adsorption from an electrolyte solution
- Differential ion dissolution from a crystal lattice
- Surface anisotropy
- Isomorphous substitution
For all liquid-liquid interfaces and most normal colloidal dispersions, Mechanism 1 is of little significance, though an exception would be metal colloid systems (e.g., gold nanoparticles). Mechanism 2 is commonly observed with carboxylic acid- and amine-containing surfaces (ex. proteins and ionic polymers) and all oxide surfaces. Lyophobic colloidal dispersions (ex. polymer lattices and APIs) fall in the Mechanism 3 category. Ionic solids (ex. silver halides and calcium carbonate) acquire a surface charge via Mechanism 4 due to unequal dissolution of their oppositely charged ions. Mechanism 5 arises because most crystal lattices are anisotropic and it is the cause of amphoteric hydroxyl groups in oxides, including silicas9. Mechanism 6 is a more extreme case of Mechanism 5 and occurs in alumino-silicate clay materials (ex. montmorillonite and vermiculite) because of the difference in valence between the Al3+ and the Si4+ ions10.
Conclusion
Colloidal systems will continue to be a part of everyday life, as they have for centuries, while progressively growing in their application potential. The pharmaceutical industry increasingly utilizes colloids, particularly when it comes to nanoparticulates. Hundreds of pharmaceutical, cosmeticeutical, and personal care products utilize some form of nanotechnology today, and many more employ slightly larger microparticles to achieve similar benefits. The challenge for traditional pharmaceutical companies is to deliver the right therapeutic to the right target with no, or minimal, side effects (ideally at reduced cost). Nanoparticles help achieve this goal through benefits such as improved bioavailability, reduced side effects, and targeted drug delivery. Nanotechnology is also versatile and can be applied to all common routes of administration, including oral, injection, transdermal, transmucosal, ocular, pulmonary, and implant.
Enhanced drug delivery is a critical area of pharmaceutical research and is one where nanoparticles have proven exceptionally useful. When designing a nanoparticulate colloidal system, a formulator must consider specific particle properties and understand how these influence the overall system. With an understanding of the established science behind nanotechnology, scientists will continue to utilize nanoparticles to improve healthcare for decades to come.
References
- Hunter, Foundations of Colloid Science, Oxford University Press, New York (1987)
- Stein, The Preparation of Dispersions in Liquids, Surfactant Science Series, Volume 58, Marcel Dekker, New York (1996).
- Zettlemoyer (ed.), Nucleation, Marcel Dekker, New York (1969).
- Buckley, Crystal Growth, John Wiley, New York (1951).
- Branden and J. Tooze, Introduction to Protein Structure, 2nd. Edition, Garland Publishing, New York (1991).
- Painter and MM. Coleman, Fundamentals of Polymer Science, Technomic Publishing Co., Lancaster, PA (1994).
- Fleer, MA. Cohen Stu- art et al, Polymers at Interfaces, Chapman & Hall, London (1993).
- Lyklema, Fundamentals of Interface and Colloid Science, Elsevier, Amsterdam (1991).
- Iler, Chemistry of Silica, Wiley-VCH Verlag, Weinheim (1979).
- van Olphen, Introduction to Clay Colloid Chemistry, Interscience, London (1963).
